
Are you aware that your diet can change the function of your genes, with an impact that might extend to your children and grandchildren? Even though the sequence of your DNA remains pretty much the same for your entire life, the way your genes work – or don’t – can be dramatically altered if you make specific epigenetic changes. These are chemical modifications that can alter the way a strand of DNA behaves, much like changing a light switch from the “off” to the “on” position.
Key Takeaways
DNA methylation is the most studied epigenetic modification, occurring when methyl groups attach to DNA (typically at CpG islands), which generally silences gene expression by preventing transcription factors from binding.
Histone modifications act as biological switches for genes through processes including acetylation (activating genes), methylation (can activate or repress genes), phosphorylation, and ubiquitination—collectively forming a complex “histone code.”
Non-coding RNAs (including microRNAs, long non-coding RNAs, and small interfering RNAs) regulate gene expression without being translated into proteins, creating intricate regulatory networks throughout the genome.
Chromatin remodeling directly alters DNA packaging structure through ATP-dependent complexes that reposition, remove, or exchange nucleosomes, making genes more or less accessible for transcription.
RNA modifications, such as N6-methyladenosine (m6A) and 5-methylcytosine (m5C), create an additional layer of epigenetic regulation by directly altering RNA behavior, thereby affecting processes ranging from RNA processing to protein translation.
Higher-order chromatin organization, including Topologically Associating Domains (TADs) and nuclear compartmentalization, creates a three-dimensional framework that controls which genes interact and are expressed based on their physical positioning within the nucleus.
Table of Contents
Understanding The Types of Epigenetic Modifications
The Foundation of Gene Regulation
A fascinating layer of biological regulation doesn’t alter your DNA sequence but dramatically changes how genes behave. Think of your DNA as the script for a play—epigenetics is like the director deciding which scenes get performed and which remain unread.
These modifications function as biological switches, toggling genes on or off in diverse ways. When you delve into nutrigenomics with your patients, understanding these mechanisms is crucial for elucidating how their diet directly impacts their genetic expression.
DNA Methylation: The Original Epigenetic Mark
DNA methylation is the granddaddy of epigenetic modifications. It occurs when methyl groups attach to specific DNA regions, typically at CpG islands (clusters of cytosine-guanine sequences). This process is facilitated by enzymes called DNA methyltransferases.
When methylation happens near a gene’s promoter region, it typically silences that gene by preventing transcription factors from binding. This is why DNA methylation is often associated with gene repression. For patients with conditions such as diabetes or cardiovascular disease, methylation patterns can be particularly relevant—dietary factors can significantly influence these patterns.
Histone Modifications: Rewriting the Instruction Manual
Histones are proteins that DNA wraps around, like thread on spools. These proteins can undergo several types of modifications:
Histone acetylation adds acetyl groups to histones, loosening DNA packaging and typically activating gene expression.
Histone methylation adds methyl groups to specific amino acids in histones, either activating or repressing genes depending on the location of the modification.
Histone phosphorylation adds phosphate groups, often related to chromosome condensation during cell division.
These modifications create what scientists call the “histone code”—a complex set of instructions that determine which genes are accessible for transcription. Many nutrients your patients consume daily, like folate, choline, and vitamins B2, B6, and B12, directly influence these histone modifications.
Non-coding RNA Regulation: The Hidden Controllers
Beyond DNA and histones, various RNA molecules that don’t code for proteins play essential roles in epigenetic regulation:
MicroRNAs (miRNAs) are tiny RNA molecules that bind to messenger RNAs, preventing protein production or triggering degradation.
Long non-coding RNAs (lncRNAs) regulate gene expression at both transcriptional and post-transcriptional levels.
Circular RNAs (circRNAs) primarily function by binding to miRNAs, thereby indirectly affecting gene expression.
These RNA-based mechanisms add another layer of complexity to gene regulation. Your patients’ dietary choices can affect the production and function of these non-coding RNAs, creating ripple effects throughout their gene expression profiles.
Chromatin Remodeling: Reshaping the Genome’s Architecture
Chromatin structure—how tightly or loosely DNA is packaged—dramatically affects which genes can be accessed. Specialized protein complexes can remodel chromatin, sliding nucleosomes (DNA-histone complexes) to expose or hide genes.
This architectural remodeling is responsive to environmental factors, including nutrients. High glycemic index diets, for instance, have been linked to specific chromatin remodeling patterns that affect gene expression related to metabolism and inflammation.
Understanding these fundamental epigenetic mechanisms provides the foundation for applying nutrigenomics in your practice. These modifications help explain why identical twins with the same DNA sequence can develop different health conditions based on their lifestyle choices—and why personalized nutrition plans based on genetic profiles can be so effective for your patients.
DNA Methylation: The Most Studied Epigenetic Mark
DNA methylation serves as the cornerstone of epigenetic research, providing crucial insights into how our genes respond to environmental factors without altering the underlying DNA sequence. This modification plays a fundamental role in regulating gene expression across various biological processes.
CpG Methylation Patterns
CpG methylation means a methyl group attaches to the 5th carbon of cytosine in CpG dinucleotides. These are regions where cytosine is followed by guanine in the DNA sequence, and CpG sites cluster in CpG islands near gene promoters. Methylation of these sites can suppress gene transcription.
Tissue-specific and cellular identity are crucial aspects of maintaining cellular dynamics in CpG methylation patterns. These patterns serve as molecular fingerprints that influence not just cell type but also cell-state identity, meaning transcriptional regulatory programs that direct cell function and differentiation. In a clinical context, we are most familiar with abnormal patterns of CpG methylation. For instance, hypermethylation of the promoters of tumor suppressor genes is intimately connected with the pathogenesis of many forms of cancer.
Non-CpG Methylation
Cytosines that are not followed by guanine (e.g., CpA, CpT, or CpC) are where non-CpG methylation occurs, and this type of methylation is abundant in pluripotent stem cells, neurons, oocytes, and glial cells. While non-CpG methylation has received less attention than its CpG counterpart, recent advancements in sequencing have bolstered our understanding of its potential to act as a repressive mark in gene regulation.
This is especially true within the brain, where non-CpG methylation appears to restrict its repressive effects to specific types of genes. As far as non-CpG methylation is concerned, its exact functions are not yet fully understood.
Histone Modifications: Chemical Tags That Control Chromatin
Histones are proteins that surround DNA, forming chromatin. Their chemical modification can loosen or tighten chromatin structure, so activating or repressing gene transcription.
Histone Acetylation
Making genes more accessible involves adding acetyl groups to histone tails, which loosens histones’ grip on DNA. This is done by histone acetyltransferases (HATs), which increase gene expression, and by histone deacetylases (HDACs), which decrease expression. HATs and HDACs are pretty special.
That’s because, unlike most proteins that are either good or bad for gene expression, HATs and HDACs act on the same molecule (the histone) but with opposite effects. So far, we’ve been talking about gene accessibility in a general sense. But different histone tails, when acetylated, have different effects on other genes. Some of these effects have been shown to have significant consequences for cell function.
Histone Methylation
Methylation of histones is a complex process that can either activate or deactivate the expression of genes. It depends on which amino acid on the histone is methylated, and more importantly, on how many methyl groups are added. For instance, methylation at H3K4 typically activates genes, while methylation at H3K9 or H3K27 represses them. Also very important is the number of methyl groups added: mono-, di-, or trimethylation. This process allows precise control over gene expression.
Histone Phosphorylation
Adding phosphate groups to histone proteins involves kinase enzymes, and these groups are often added to serine or threonine sites. This is the best-understood form of histone modification. It directly affects the functioning of chromatin, particularly in two key areas: (1) signaling when and where DNA repair is needed, and (2) regulating gene expression, for which the phosphorylated state is often enhanced by other forms of protein “decoration” like acetylation. In both cases, the addition of a phosphate group has a direct effect on chromatin structure that helps program the cell’s function.
Histone Ubiquitination
Ubiquitination of histones is unlike any of the other known histone modifications because whole ubiquitin proteins, instead of small chemical groups, are attached to the histones. This is a huge change, and the attachment of ubiquitin does not appear to be a direct signal that affects gene expression. Ubiquitination of histones appears to be crucial for DNA repair, as well as for maintaining chromatin stability.
Together, these four types of histone modifications form a “histone code”—a complex, dynamic system that fine-tunes gene expression across the genome. As nutrigenomics research advances, understanding how dietary factors influence these modifications offers exciting possibilities for personalized nutrition approaches to health and condition prevention.

Chromatin Remodeling: Structural Changes in DNA Packaging
Chromatin remodeling represents one of the most fascinating epigenetic mechanisms that controls gene expression through dynamic changes in DNA packaging. Unlike DNA methylation or histone modifications, chromatin remodeling directly alters the physical structure of chromatin, changing how tightly DNA is wrapped around histones and affecting which genes are accessible to transcription machinery.
ATP-Dependent Chromatin Remodeling
ATP-dependent chromatin remodeling complexes serve as molecular machines that use energy from ATP hydrolysis to restructure, mobilize, or eject nucleosomes. These powerful complexes fall into several families, including the SWR1 family, which plays a critical role in genome maintenance. When these remodelers get to work, they can:
Reposition nucleosomes along DNA strands, effectively sliding them to new locations to either expose or hide regulatory regions.
Eradicate histone octamers from specific DNA segments to create nucleosome-free regions where transcription factors can bind.
Exchange canonical histones with variant histones (like replacing standard H2A/H2B dimers with specialized variants), which alters nucleosome stability and function
The beauty of ATP-dependent remodeling lies in its precision and responsiveness. These complexes don’t just randomly shuffle nucleosomes—they respond to specific cellular signals and work in concert with other epigenetic mechanisms to fine-tune gene expression patterns. For instance, when environmental factors trigger stress responses, specific remodeling complexes can quickly reorganize chromatin to activate genes needed for cellular adaptation.
Nucleosome Positioning
Nucleosome positioning represents another critical aspect of chromatin remodeling, determining which DNA sequences are accessible to the transcription machinery. This process involves:
De novo assembly of nucleosomes at specific genomic locations
Precise positioning of nucleosomes relative to specific DNA sequence features, such as transcription start sites.
Establishment of regular spacing between nucleosomes, which affects higher-order chromatin folding
Nucleosome positioning is guided by DNA sequences, with some sequences favoring nucleosome formation and others resisting it. Chromatin remodelers shape these patterns to regulate genes. Nucleosome positioning is stable enough to preserve cell identity but can change during cell differentiation or in response to environmental stress, enabling rapid gene activation. In nutrigenomics, diet influences chromatin remodeling, demonstrating how gene responses can be affected by personalized nutrition based on genetic profiles, making them more effective than one-size-fits-all dietary recommendations.
Non-Coding RNA-Mediated Modifications: Regulatory Molecules
Non-coding RNAs serve as potent epigenetic regulators that influence gene expression without being translated into proteins. These molecules work in conjunction with DNA methylation and histone modifications to create complex regulatory networks that fine-tune genetic expression.
MicroRNA Regulation
MicroRNA regulation is a process involving tiny non-coding RNAs that regulate gene expression by fine-tuning how much a gene is expressed rather than turning it fully on or off. They bind to target mRNAs, leading to their degradation or blocking their translation. This silences genes without altering their DNA and enables a single miRNA to influence hundreds of genes, thereby playing a critical role in cellular regulation.
In nutrigenomic applications, certain dietary compounds, such as polyphenols in green tea and resveratrol in grapes, have been shown to alter miRNA expression profiles. For healthcare practitioners working with personalized nutrition plans, understanding these miRNA-nutrient interactions provides another layer of precision when tailoring dietary recommendations to genetic profiles.
Long Non-Coding RNA Functions
Long non-coding RNAs (lncRNAs) are RNA molecules over 200 nucleotides long that don’t make proteins but regulate gene expression in many ways. They can recruit protein complexes, act as scaffolds, trap regulatory molecules, influence mRNA stability, or guide epigenetic modifiers to specific genome regions.
LncRNAs play key roles in vascular health and susceptibility to conditions like cardiovascular and metabolic diseases. Understanding how diet affects lncRNA expression could help explain why people respond differently to the same nutritional plans, aiding in the development of personalized nutrition strategies.
Small Interfering RNA Mechanisms
The small interfering RNA (siRNA) is a double-stranded RNA molecules that target specific messenger RNA (mRNA) with high precision. They join the RNA-induced silencing complex (RISC), which utilizes one strand of the siRNA to locate and bind to matching mRNA sequences. RISC then cuts the target mRNA, halting protein production. Additionally, siRNAs can affect epigenetic changes by modifying chromatin at gene promoters, leading to transcriptional repression.
What makes siRNAs particularly relevant to nutrigenomics is their involvement in mediating epigenetic regulation by affecting histone modifications and DNA methylation at specific genomic loci. Some bioactive food compounds have been shown to influence siRNA pathways, potentially explaining how certain diets might epigenetically reprogram gene expression patterns. For practitioners exploring cutting-edge nutrigenomic approaches, understanding these siRNA-mediated mechanisms offers insights into how dietary interventions might be designed to target specific epigenetic pathways in personalized nutrition programs.
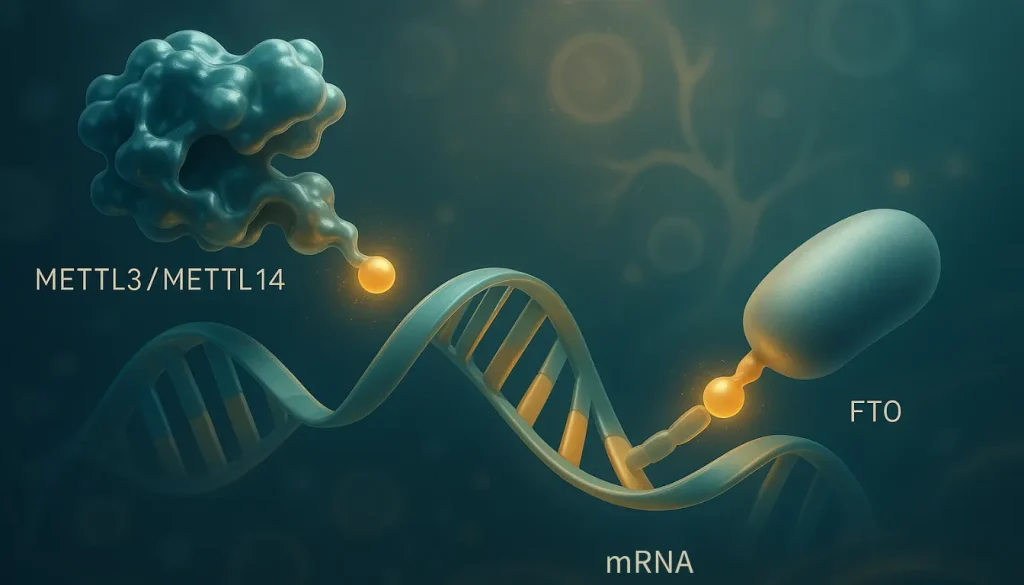
RNA Modifications: Direct Chemical Changes to RNA Molecules
RNA modifications represent a fascinating frontier in epigenetics, where chemical alterations directly impact RNA function without changing the underlying genetic code. These modifications create an additional layer of complexity in gene regulation that’s increasingly relevant to nutrigenomic approaches in healthcare.
N6-Methyladenosine (m6A) Modification
The m6A modification is the most abundant internal modification found in eukaryotic messenger RNA (mRNA). It’s like a molecular switch that can be flipped on or off, affecting how RNA behaves in the cell. This modification occurs when a methyl group attaches to the nitrogen at position 6 of adenosine, controlled by a complex interplay of “writer” enzymes, such as METTL3 and METTL14, that add the methyl group, and “eraser” enzymes, like FTO, that remove it.
What makes m6A modifications particularly interesting for healthcare practitioners is their dynamic and reversible nature. Unlike DNA methylation, which tends to be more stable, m6A marks can change rapidly in response to environmental cues, including dietary factors. For instance, specific nutrients can influence the activity of m6A-modifying enzymes, potentially affecting how genes are expressed in response to what your patients eat.
The m6A modification influences nearly every aspect of RNA metabolism—from how RNA is processed inside the nucleus to how efficiently it’s translated into proteins in the cytoplasm. It has been linked to important biological processes, including cell differentiation, embryonic development, and stress responses—all relevant factors when designing personalized nutrition plans.
5-Methylcytosine in RNA
5-methylcytosine (m5C) modification, known for its role in DNA, is gaining attention in RNA. This occurs when a methyl group attaches to the fifth position of cytosine in RNA, impacting RNA stability, structure, and protein interactions, which in turn influence gene expression.
Enzymes like NSUN proteins and DNMT2 add these methyl groups in response to cellular signals, including nutritional changes. For nutrigenomics, understanding m5C modifications facilitates the connection between dietary interventions and gene expression and health outcomes, as they are involved in stress responses and metabolism.
Research indicates that bioactive food compounds can alter m5C patterns, suggesting the dietary impact on health at the molecular level. Advanced nutrigenomic testing focusing on RNA modifications could refine personalized nutrition plans, going beyond traditional DNA analysis. Understanding these mechanisms helps interpret test results and explain how diet impacts gene expression.
Higher-Order Chromatin Structure: Three-Dimensional Organization
Beyond individual epigenetic modifications, your DNA is organized into complex three-dimensional structures that dramatically influence gene expression. This higher-order organization creates a dynamic framework where genes can be activated or silenced based on their physical positioning within the nucleus.
Topologically Associating Domains
Topologically Associating Domains (TADs) are large chromatin regions where DNA sequences interact primarily within the same domain, rarely crossing into other domains. They regulate gene expression by controlling enhancer-promoter interactions, ensuring that enhancers precisely target specific promoters. TAD boundaries, marked by proteins like CTCF and cohesin, act as insulating barriers.
Disruptions in TADs can misregulate gene expression, leading to developmental disorders and diseases by allowing enhancers to activate unintended genes. Epigenetic marks, such as histone modifications, influence the formation and maintenance of TADs. These marks can be affected by diet, subtly altering TAD structures and impacting how genes respond to nutrition over time.
Nuclear Compartmentalization
The nucleus is a highly organized structure that influences gene activity through nuclear compartmentalization, an additional layer of epigenetic regulation. The genome is divided into A and B compartments: A compartments contain euchromatin with active genes (e.g., marked by H3K9ac), while B compartments hold heterochromatin with repressed genes (e.g., marked by H3K27me3).
Specialized structures, such as nuclear speckles (active genes) and the nuclear lamina (silenced genes), further influence gene expression based on a gene’s position. This organization is dynamic—genes can move between compartments in response to signals, such as nutrients, altering their expression patterns.
For nutrigenomics, understanding how dietary inputs affect this spatial genome arrangement is crucial. It reveals how nutrition can influence gene expression, enabling the development of personalized nutritional plans based on individual genetic profiles.
Conclusion
Epigenetic modifications represent a fascinating bridge between your environment and genetic expression. From DNA methylation to histone modifications, these cellular mechanisms respond to what you eat and how you live.
The intricate dance of chromatin remodeling, non-coding RNAs, and RNA modifications shows just how adaptive your body is at the molecular level. Your lifestyle choices create ripples through these systems, affecting not just your health but potentially future generations too.
Understanding these modifications gives you unprecedented power to influence your genetic destiny through informed dietary choices. As research in nutrigenomics advances, you’ll have even more tools to optimize your health based on your unique genetic profile rather than following one-size-fits-all advice.
Frequently Asked Questions
How does diet influence gene expression?
Diet influences gene expression through epigenetic modifications like DNA methylation, histone modifications, and changes in non-coding RNAs. Nutrients can add or remove chemical tags on DNA or histones, altering how genes are accessed and read. These modifications can activate or silence genes, affecting metabolism, inflammation, and disease risk without changing the underlying DNA sequence.
What are histone modifications?
Histone modifications are chemical changes to histone proteins around which DNA wraps. These include acetylation, methylation, phosphorylation, and ubiquitination of histone tails. These modifications alter chromatin structure, making genes either more or less accessible for transcription. The specific pattern of modifications creates a “histone code” that helps regulate gene expression in response to environmental factors like diet.
How do non-coding RNAs affect gene expression?
Non-coding RNAs regulate gene expression without being translated into proteins. MicroRNAs (miRNAs) bind to messenger RNAs and prevent protein production. Long non-coding RNAs (lncRNAs) influence chromatin structure and recruit regulatory proteins. Small interfering RNAs (siRNAs) participate in RNA interference. Dietary compounds can alter the expression of these regulatory RNAs, affecting cellular functions and metabolic pathways.
Can epigenetic changes be passed to future generations?
Yes, some epigenetic changes can be passed on to future generations, a phenomenon known as transgenerational epigenetic inheritance. Modifications that occur in germ cells (eggs or sperm) due to dietary habits or environmental exposures can potentially affect offspring. This explains how parents’ lifestyle choices, including diet, might influence their children’s health predispositions, even without changes to the genetic code.
What is chromatin remodeling?
Chromatin remodeling is the dynamic process of altering chromatin structure to control gene accessibility. ATP-dependent complexes reposition nucleosomes, create nucleosome-free regions, and exchange histones to regulate gene expression. This process responds to environmental signals, including dietary factors, and determines which genes are available for transcription, playing a crucial role in cellular adaptation to nutritional changes.
What are RNA modifications?
RNA modifications are direct chemical changes to RNA molecules that affect their function. The most common is N6-methyladenosine (m6A), which acts as a molecular switch influencing RNA stability, translation, and localization. Another important modification is 5-methylcytosine (m5C). These modifications respond to environmental cues, including diet, and provide an additional layer of gene regulation beyond DNA-level changes.
What are Topologically Associating Domains (TADs)?
TADs are regulatory units of three-dimensional chromatin structure that control gene expression by facilitating or restricting enhancer-promoter interactions. They create boundaries that compartmentalize the genome into functional regions. TADs can be influenced by epigenetic marks and dietary factors, affecting which genes are expressed. Disruption of TAD boundaries can lead to inappropriate gene activation or silencing, contributing to disease development.
How can understanding epigenetics improve personalized nutrition?
Understanding epigenetics can significantly improve personalized nutrition by revealing how individual genetic profiles respond to specific dietary components. This knowledge allows healthcare practitioners to develop targeted nutrition plans that optimize gene expression patterns, potentially preventing disease development and improving health outcomes. Personalized approaches based on epigenetic insights are likely more effective than generic dietary recommendations.

